- Highest scanning speed, accuracy, and resolution
Vieworks, a leading provider of imaging solution, has unveiled the slide scanner 'LUCEON', raising expectations for growth in the diagnostic equipment market.
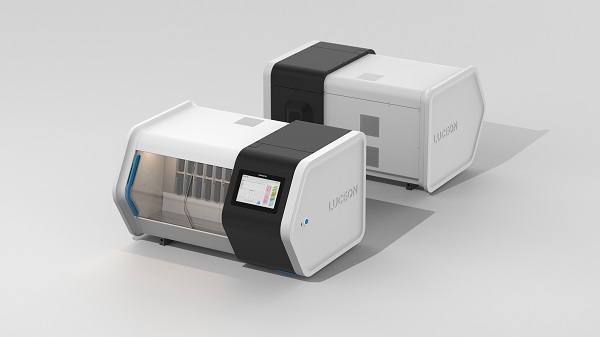
Vieworks announced that it participated in the Korean Society of Pathology Fall Conference held at The-K Hotel in Seoul from the 25th to the 27th and announced the prototype of slide scanner 'LUCEON', a core equipment of digital pathology diagnosis system, and related patented technologies.
Slide scanners are core equipment for digital pathology diagnostics that are rapidly developing in the United States and Europe, and a new market was opened in 2017 when the U.S. Food and Drug Administration (FDA) certified them as in vitro diagnostic equipment for the first time.
Vieworks started developing the slide scanner 'LUCEON' in 2021 to lead the spread of digital pathology systems based on its experience in leading optical technology in the medical and bio imaging fields.
Vieworks' new LUCEON is an ultra-fast digital pathology scanner that reconstructs glass tissue slides into high-resolution Whole Slide Images (WSI). The introduction of digital pathology diagnostics using WSI will enable semi-permanent storage of patients' tissue slides, reducing space constraints and increasing the accuracy of diagnosis through consultation and telemedicine.
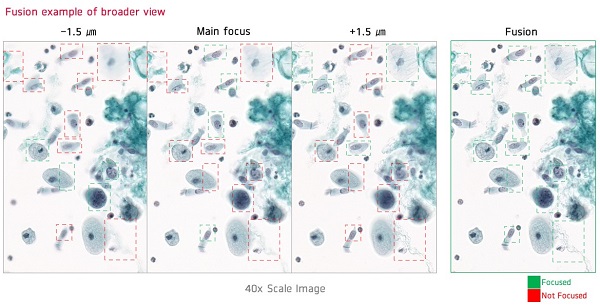
LUCEON's core strengths are its unrivaled WSI generation speed and high resolution.
With up to 510 tissue slides loaded at a time, LUCEON can generate 83 WSIs (15x15mm) per hour, the fastest of any scanner on the market. In addition, the resolution of the WSI is dramatically increased by applying a proprietary technology that captures three images with different focal lengths in a single scan, selecting and compositing only the sharpest areas.
The development of 'LUCEON' was based on active collaboration with professors in the pathology departments of large domestic hospitals. Vieworks sincerely reflects the requirements of pathologists and enhances the perfection of its products through long product verification, and earlier this month, it obtained a manufacturing license for 'cell and tissue pathology inspection device' from the Korean Ministry of Food and Drug Safety.